|
|
|
Research programs Research
Interests Techniques Representative Projects FAQ
FAQ: Ansewrs from
Wikipedia, the free encyclopedia
----------------------------------------------------------------
What is
medicinal chemistry?
|
Medicinal or pharmaceutical chemistry
is a highly interdisciplinary science combining organic chemistry with
pharmacy, biochemistry and many other branches of science. Medicinal
chemistry involves the design, synthesis, identification and development
of new chemical entities suitable for therapeutic use (pharmaceutical
drugs).
It also includes study of existing drugs, their biological
properties, and their quantitative structure-activity relationships the
(QSAR). Pharmaceutical chemistry is focused on quality aspects of
medicines and aims to assure fitness for the purpose of medicinal
products.
top
|
----------------------------------------------------------------
What is
chemotherapy?
|
Chemotherapy is the use of chemical substances to treat disease. In its
modern-day use, it refers primarily to cytotoxic drugs used to treat
cancer.
In its non-oncological use, the term may also refer to antibiotics (antibacterial
chemotherapy). In that sense, the first modern chemotherapeutic agent
was Paul Ehrlich's arsphenamine, an arsenic compound discovered in 1909
and used to treat syphilis.
Anther example is the penicillin G discovered by Alexander Fleming.
top
|
----------------------------------------------------------------
What is
oncology?
|
Oncology is the branch of medicine that studies tumors (cancer) and seeks to
understand their development, diagnosis, treatment and prevention.
A physician who practices oncology is an oncologist. The term
originates from the Greek onkos meaning
bulk, mass, or tumor and the suffix -ology, meaning "study
of".
top
|
----------------------------------------------------------------
What is
cancer?
|
Cancer is a class of diseases or disorders
characterized by uncontrolled division of cells and the ability of these
to spread, either by direct growth into adjacent tissue through invasion,
or by implantation into distant sites by metastasis (where cancer
cells are transported through the bloodstream or lymphatic system).
Cancer may affect people at all ages, but risk tends to increase with
age. It is one of the principal causes of death in.
Most cancers can be treated and some cured, depending on the specific
type, location, and stage. Once diagnosed, cancer is usually treated with
a combination of surgery, chemotherapy and radiotherapy.
As research develops, treatments are becoming more specific for the
type of cancer pathology. Drugs that target specific cancers already
exist for several types of cancer. If untreated, cancers may eventually
cause illness and death, though this is not always the case.
The unregulated growth that characterizes cancer is caused by damage
to DNA, resulting in mutations to genes that encode for proteins
controlling cell division.
Many mutation events may be required to transform a normal cell into
a malignant cell. These mutations can be caused by radiation, chemicals
or physical agents that cause cancer, which are called carcinogens.
Additionally, many forms of cancer are associated with exposure to
environmental factors such as tobacco smoke, radiation, alcohol, and
certain viruses. Some risk factors can be avoided or reduced.
top
|
----------------------------------------------------------------
What is
tumor?
|
Tumor or tumour
(via Old French tumour from Latin tumor
"swelling") is primarily used to denote abnormal growth of tissue.
This growth can be either malignant or benign. It is similar in meaning
to a neoplasm. For malignant tumors specifically, see cancer.
top
|
----------------------------------------------------------------
What is drug?
|
A drug is any chemical
or biological substance,
synthetic or non-synthetic, that when taken into the organism's body,
will in some way alter the functions of that organism.
This broad definition can be taken to include such substances as
food. In these cases the word "drug"
is usually used to refer specifically to medicine. Many natural
substances such as beers, wines blur the line between food and drugs, as
when ingested they affect the functioning of both mind and body.
Drugs are usually distinguished from endogenous biochemicals
(substances originate from within an organism) by being introduced from
outside the organism.
For example, insulin is a hormone that is synthesized in the body; it
is called a hormone when it is synthesized by the pancreas inside the
body, but if it is introduced into the body from outside, it is called a
drug.
top
|
----------------------------------------------------------------
What is cytostatik drug?
|
Cytostatic drugs (or Cytostatika, from the
Greek Cyto = cell and statics = continue) are
natural or synthetic substances, which restrain cell growth and/or the
cell division.
They are used particularly for the treatment of cancer
(chemotherapy). Beside the classical cytostatic drugs, further substances
as hormones, therapeutic monoclonal anti-bodies and so-called “small
molecules" as proteasome inhibitors are used nowadays for the
treatment of tumor illnesses.
top
|
----------------------------------------------------------------
What is drug
delivery?
|
Drug delivery is a term that refers to the delivery of a pharmaceutical compound
to humans or animals. Most common methods of delivery include the
preferred non-invasive oral (through the mouth), nasal, pneumonial (inhalation) and rectal routes.
Many medications, however, can not be
delivered using these routes because they might be susceptible to
degradation or are not incorporated efficiently. For this reason many protein and peptide drugs have to be
delivered by injection. For example, many immunizations are based on the
delivery of protein drugs and are often done by injection.
Current efforts in the area of drug delivery include the development
of targeted delivery in which the drug is only active in the target area
of the body (for example, in cancerous tissues) and sustained release
formulations in which the drug is released over a period of time in a
controlled manner from a formulation.
top
|
----------------------------------------------------------------
What is a
drug carrier?
|
Drug carriers are substances that serve as mechanisms to improve the delivery and the
effectiveness of drugs. Drug carriers are used in sundry drug delivery
systems such as:
·
controlled-release technology to
prolong in vivo drug actions;
·
decrease drug metabolism, and
·
reduce drug toxicity.
Carriers are also used in designs to increase the effectiveness of
drug delivery to the target sites of pharmacological actions.
top
|
----------------------------------------------------------------
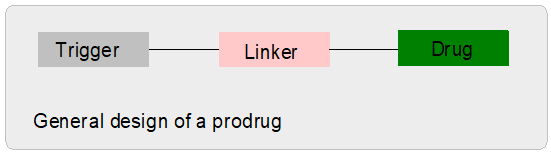
What is a
prodrug?
|
A prodrug is a pharmacological
substance (drug) which is administered in an inactive (or significantly
less active) form. Once administered, the prodrug is metabolised
in vivo into the active compound.
An example of prodrug: Chloramphenicol succinate ester is
used as intravenous prodrug of chloramphenicol, because pure
chloramphenicol does not dissolve in water.
top
|
----------------------------------------------------------------
What is in
vivo?
|
In
vivo (Latin: (with)in the living) means that which takes place inside an
organism. In science, in vivo refers to experimentation done
in or on the living tissue of a whole, living organism as opposed to a
partial or dead one.
Animal testing and clinical trials are forms of in vivo
research.
top
|
----------------------------------------------------------------
What is in
vitro?
|
In vitro (Latin: (with)in the glass) refers to
the technique of performing a given experiment in a test tube, or,
generally, in a controlled environment outside a living organism.
top
|
----------------------------------------------------------------
What is a
macromolecule?
|
The literal definition of the term macromolecule implies any large molecule. In the context of
science and engineering, the term may be applied to conventional polymers
and biopolymers (such as DNA) as well as non-polymeric molecules with
large molecular mass such as lipids or macrocycles. However, other large
networks of atoms, such as metallic covalent networks or fullerenes, are
not generally described as macromolecules.
The use of the term macromolecule varies subtly from discipline to
discipline. From the strict perspective of chemistry, a
"molecule" comprises a number of atoms linked by covalent
bonds. In biology and biochemistry, however, the term macromolecule may
refer to aggregates of two or more macromolecules held together by
intermolecular forces rather than covalent bonds but which do not readily
dissociate.
According to the recommended IUPAC definition the term macromolecule
as used in polymer science refers only to a single molecule. For example,
a single polymeric molecule is appropriately described as a
"macromolecule" or "polymer molecule" rather than a
"polymer", which suggests a substance composed of
macromolecules.
top
|
----------------------------------------------------------------
What is
albumin?
|
Albumin (Latin: albus,
white) refers generally to any protein with water solubility, which is
moderately soluble in concentrated salt solutions, and experiences heat coagulation
(protein denaturation: denaturation
is the alteration of a protein shape through some form of external stress
[for example, by applying heat, acid or alkali], in such a way that it
will no longer be able to carry out its cellular function.).
Substances containing albumin, such as egg white, are called albuminoids.
top
|
----------------------------------------------------------------
What is human
serum albumin?
|
Human
serum albumin is the most abundant protein in human
blood plasma. It is produced in the liver. The reference range for
albumin concentrations in blood is 30 to 50 g/L (3.0 to 5.0 g/dL). It has
a serum half-life of approximately 20 days. It has a molecular mass of 67
kDa.
top
|
----------------------------------------------------------------
What is SAR?
|
Structure-activity relationship (SAR) is the
traditional practices of medicinal chemistry which try to modify the
effect or the potency {ie.
activity} of bioactive chemical compound by modifying its
chemical structure.
Medicinal chemists were using the chemical techniques of synthesis to
insert new chemical groups into the biomedical compound and test the
modifications in its biological effect. This process enabled them to
determine the responsible chemical groups for evoking the biological
effect in the organism.
top
|
----------------------------------------------------------------
What is MDR?
|
Multidrug resistance is the ability of pathologic cells to withstand chemicals that are
designed to aid in the eradication of such cells. These pathologic cells
include bacterial and neoplastic (tumor) cells. Cancer cells also have
the ability to become resistant to multiple different drugs, and share
many of the same mechanisms:
·
Increased efflux of drug (as by
P-glycoprotein, multidrug resistance-associated protein, lung resistance
related protein, and breast cancer resistance protein)
·
Enzymatic deactivation (i.e.
glutathione conjugation)
·
Decreased permeability (drugs can't
enter the cell)
·
Altered binding-sites
·
Alternate metabolic pathways (the
cancer compensates for the effect of the drug).
top
|
----------------------------------------------------------------
What is Pgp?
|
P-glycoprotein (abbreviated as P-gp or Pgp) is a well
characterized human ABC-transporter (ATP-binding cassette)
of the MDR/TAP (Transporter associated with antigen processing)
subfamily.
It is extensively distributed and expressed in normal cells such as
those lining the intestine, liver cells, renal proximal tubular cells and
capillary endothelial cells comprising the blood-brain barrier. P-gp is also called ABCB1, ATP-binding cassette
sub-family B member 1, MDR1, P-glycoprotein and PGY1.
A frequent cause of drug resistance results from an elevated
expression of the cell-membrane transporter efflux pump Pgp that functions by increasing the efflux of
cytotoxic drugs from cancer cells and allows them to survive by lowering
the intracellular concentrations of the drugs. A lot of specific P-gp modulators have been developed in order to
circumvent MDR:
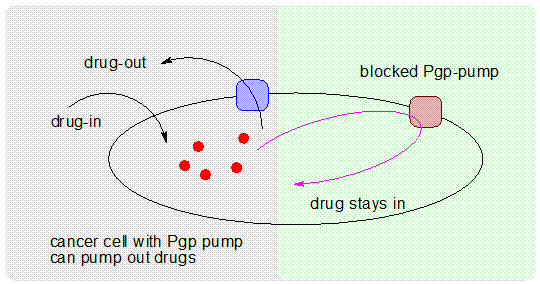
top
|
----------------------------------------------------------------
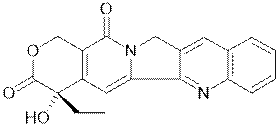
What is Camptothecin (CPT)?
|
Camptothecin is a plant secondary metabolite used as an anti-cancer drug that
damages DNA, leading to the destruction of the cell.
It comes from Camptotheca
acuminata, a deciduous tree found in
southern China.
Stem woods of Nothopodytes foetida (previously known as Mappia
foetida) found in the western ghats of India are an even better
source of camptothecin.
top
|
|
-------------------------------------------------------------
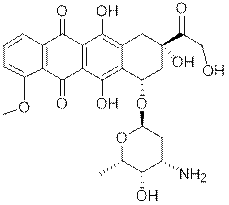
What is Doxorubicin (dox)?
|
Doxorubicin (trade name Adriamycin)
or hydroxyldaunorubicin
is a DNA-interacting drug widely used in chemotherapy. It is an
anthracycline antibiotic and structurely
closely related to daunomycin, and also intercalates DNA. It is commonly
used in the treatment of a wide range of cancers.
The drug is administered by injection,
and may be sold under the brand names Adriamycin PFS,
Adriamycin RDF, or Rubex.
|
|
|
Doxil is a liposome-encapsulated dosage form of doxorubicin made by Ben
Venue Laboratories for Johnson & Johnson. The main benefits of this
form are a reduction in cardiotoxicity.
top
|
----------------------------------------------------------------
What is taxane?
|
The taxanes
are diterpenes produced by the plants of the genus Taxus (yews).
As their name suggests, they were first derived from natural sources, but
some have been synthesized artificially. Taxanes
include paclitaxel and docetaxel. Paclitaxel was originally derived from
the Pacific yew tree. Taxanes have been used to
produce various chemotherapy drugs.
top
|
----------------------------------------------------------------
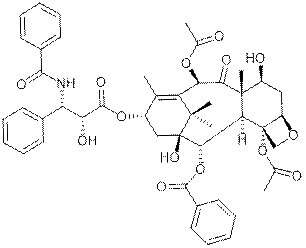
What is
Taxol?
|
Paclitaxel is a drug used in the treatment of cancer. It was discovered at
Research Triangle Institute (RTI) in 1967 when Monroe E. Wall and Mansukh
C. Wani isolated the compound from the bark of
the Pacific yew tree, Taxus brevifolia, and
noted its antitumor activity in a broad range of rodent tumors. By 1970,
the two scientists had determined the structure of paclitaxel. Paclitaxel
has since become an effective tool of doctors who treat patients with
lung, ovarian, breast cancer, and advanced forms of tumors. .
|
|
|
It is sold under the trade name Taxol. Together with docetaxel,
it forms the drug category of the taxanes.
top
|
----------------------------------------------------------------
What is
spacer?
|
A Molecular spacer or simply a spacer in chemistry is any flexible part of a molecule
providing a connection between two other parts of a molecule.
top
|
----------------------------------------------------------------
What is
dendrimer?
|
Dendrimers are repeatedly branched molecules.
top
|
----------------------------------------------------------------
What is
sesquiterpene lactone?
|
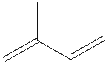
Sesquiterpene lactones are a class of chemical found in many plants that can cause allergic
reactions. Some plants containing these compounds are: Artichoke.
Sesquiterpenes are a class of terpenes that consist of three isoprene units and
have the molecular formula C15H24.
|
Isoprene
|
|
Like monoterpenes (monoterpenes are a class of
terpenes that consist of two isoprene units and have the molecular
formula C10H16), sesquiterpenes may be acyclic or
contain rings, including many unique combinations.
A lactone is a cyclic ester in organic chemistry
Terpenes are a large and varied class of hydrocarbons, produced primarily by a
wide variety of plants, particularly conifers, though also by some
insects such as swallowtail butterflies.
Terpenes are derived biosynthetically
from units of isoprene, which has the molecular formula C5H8.
The basic molecular formulas of terpenes are multiples of that, (C5H8)n where n is the number of
linked isoprene units. This is called the isoprene rule or the C5
rule.
top
|
----------------------------------------------------------------
What is NF-κB?
|
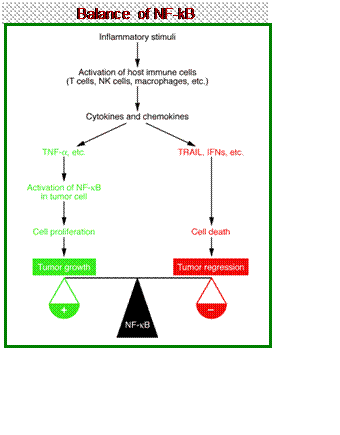
NF-κB (nuclear factor-kappa B)
is a protein complex which is a transcription factor*. It is found in
all cell types and is involved in cellular responses to stimuli such as
stress, cytokines, free radicals, ultraviolet irradiation, and bacterial
or viral antigens. NF-κB plays a key role
in regulating the immune response to infection. Consistent with this
role, incorrect regulation of NF-κB has
been linked to cancer, inflammatory and autoimmune diseases, septic
shock, viral infection and improper immune development. NF-κB has also been implicated in processes of
synaptic plasticity and memory.
|
|
NF-κB
is widely used by eukaryotic cells as a regulator of genes that control
cell proliferation and cell survival. As such, many different types of
human tumors have misregulated NF-κB: that is, NF-κB
is constitutively active. Active NF-κB
turns on the expression of genes that keep the cell proliferating and
protect the cell from conditions that would otherwise cause it to die. In
tumor cells, NF-κB is active either due to
mutations in genes encoding the NF-κB
transcription factors themselves or in genes that control NF-κB activity (such as IkB
genes); in addition, some tumor cells secrete factors that cause NF-κB to become active.
|
|
|
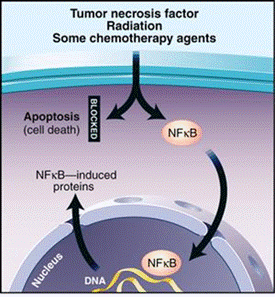
NF-κB's Role in Cancer and Other
Diseases
|
|
Blocking NF-κB
can cause tumor cells to stop proliferating, to die, or to become more
sensitive to the action of anti-tumor agents. Thus, NF-κB is the subject of much active research among
pharmaceutical companies as a target for anti-cancer therapy.
Because NF-κB
controls many genes involved in inflammation, it is not surprising that
NF-κB is found to be chronically active in
many inflammatory diseases, such as inflammatory bowel disease,
arthritis, sepsis, among others.
Many natural products (including
anti-oxidants) that have been promoted to have anti-cancer and
anti-inflammatory activity have also been shown to inhibit NF-κB. Additionally, a lot of work has been done to
develop agents that can block NF-κB for
therapeutic purposes.
|
|
|
* Transcription factor is a protein that works in concert with
other proteins to either promote or suppress the transcription**of genes.
** Transcription is the process through which a DNA sequence is
enzymatically copied by an RNA polymerase to produce a complementary RNA.
In other words, it is the transfer of
genetic information from DNA into RNA.
top
|
|
|
|
----------------------------------------------------------------
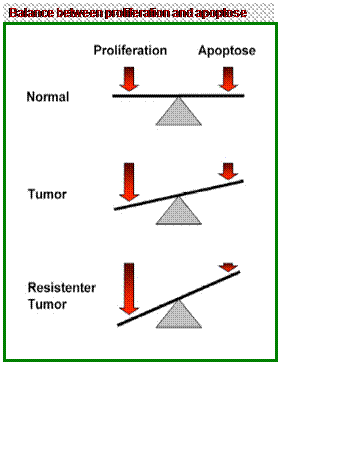
What is apoptosis?
|
Apoptosis is a process of deliberate life relinquishment by a cell in a
multicellular organism. It is one of the main types of programmed cell
death (PCD), and involves an orchestrated series of biochemical events
leading to a characteristic cell morphology (outward appearance [shape,
structure, color and pattern] and component parts) and death.
|
|
The apoptotic process is executed in
such a way as to safely dispose of cell corpses and fragments.
It is to a certain extent „a suicide
program of “individual biological cells. This can be influenced from the
outside lively (approximately by immune cells) or due to cell internal
processes to be released (for instance after strong damage of the heiress
formation).
Apoptosis is carried out in an orderly
process that generally confers advantages during an organism's life
cycle. Research on apoptosis has increased and defective apoptotic
processes have been implicated in an extensive variety of diseases
including cancer.
|
|
|
top
|
----------------------------------------------------------------
What is
plasmin?
|
Plasmin is an important enzyme (EC 3.4.21.7)
present in blood that degrades many blood plasma proteins, most notably
fibrin clots. The degradation of fibrin is termed fibrinolysis.
top
|
----------------------------------------------------------------
What is
cathepsin?
|
A cathepsin is one of a family of proteases, a type of protein
that breaks apart other proteins, found in many types of cells including
those in all animals. There are approximately a dozen members of this family,
which are distinguished by their structure and which proteins they
cleave. Most of the members become activated at the low pH found in
lysosomes. Thus, the activity of this family lies almost entirely within
those organelles.
Cathepsins have a vital role in
mammalian cellular turnover, e.g. bone resorption. They degrade
polypeptides and are distinguished by their substrate specificites.
top
|
What is toll-like receptor (TLR)?
|
Toll-like receptors (TLRs) are a class
of single membrane-spanning non-catalytic receptors that recognize
structurally conserved molecules derived from microbes once they have
breached physical barriers such as the skin or intestinal tract mucosa,
and activate immune cell responses. They are believed to play a key role
in the innate immune system.
TLRs are a type of pattern
recognition receptors (PRRs) and recognize molecules that are broadly
shared by pathogens but distinguishable from host molecules, collectively
referred to as pathogen-associated molecular patterns (PAMPs). However,
there are some exceptions to this general rule. TLRs are present in
vertebrates (including fish, amphibians, reptiles and birds and mammals)
as well as in invertebrates (such of the insect Drosophila where they have
been extensively studied). Molecular building blocks of the TLRs are
represented in bacteria and in plants, and in the latter kingdom, are
well known to be required for host defense against infection. The TLRs
thus appear to be one of the most ancient, conserved components of the
immune system.
top
|
----------------------------------------------------------------
What is immune system?
|
An immune system is a collection of mechanisms within an organism that protects against
infection by identifying and killing pathogens and tumor cells. It
detects pathogens ranging from viruses to parasitic worms and
distinguishes them from the organism's normal cells and tissues.
Detection is complicated as pathogens [pathogen or infectious
agent is a biological agent that causes disease or illness to its
host] adapt and evolve new ways to successfully infect the host organism.
top
|
----------------------------------------------------------------
What is lipid?
|
Lipids are class of hydrocarbon-containing
organic compounds. Lipids are categorized by the fact that they have
complicated solvation properties, giving rise to lipid polymorphism.
Lipid molecules have these properties because they consist largely of
long hydrocarbon tails which are lipophilic in nature as well as polar
head groups (e.g. phosphate-based functionality, and/or inositol based functionality). In living organisms,
lipids are used for energy storage, serve as the structural components of
cell membranes, and constitute important signalling
molecules. Although the term lipid is often used as a synonym for fat,
the latter is in fact a subgroup of lipids called triglycerides and
should not be confused with the term fatty acid.
top
|
----------------------------------------------------------------
What is protein?
|
Proteins are large organic compounds made of amino acids arranged in a linear
chain and joined together by peptide bonds between the carboxyl and amino
groups of adjacent amino acid residues. The sequence of amino acids in a
protein is defined by a gene and encoded in the genetic
code. Although this genetic code specifies 20 "standard" amino
acids, the residues in a protein are often chemically altered in
post-translational modification: either before the protein can function
in the cell, or as part of control mechanisms. Proteins can also work
together to achieve a particular function, and they often associate to
form stable complexes.
top
|
----------------------------------------------------------------
What is lipoprotein?
|
A lipoprotein is a biochemical assembly that contains both proteins
and lipids. The lipids or their derivatives may be covalently or
non-covalently bound to the proteins. Many
enzymes, transporters, structural proteins, antigens, adhesins and toxins
are lipoproteins.
Examples include the high density and low density lipoproteins of the blood, the
transmembrane proteins of the mitochondrion and the chloroplast, and
bacterial lipoproteins.
top
|
----------------------------------------------------------------
What is polysaccharide?
|
Polysaccharides are relatively complex carbohydrates. They are polymers made up of
many monosaccharides joined together by glycosidic
linkages. They are therefore very large, often branched, molecules. They
tend to be amorphous, insoluble in water, and have no sweet taste
top
|
----------------------------------------------------------------
What is
lipopolysaccharide?
|
Lipopolysaccharide (LPS) is a large molecule consisting of
a lipid and a polysaccharide (carbohydrate) joined by a covalent bond.
top
|
----------------------------------------------------------------
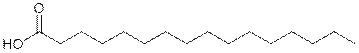
What is palmitic acid?
|
Palmitic acid, or hexadecanoic acid, is one of the most common
saturated fatty acids
|
|
|
[fatty acid is a carboxylic acid often
with a long unbranched aliphatic chain, which is either saturated or
unsaturated] found in animals and plants.
As its name indicates, it is a major
component of the oil from palm trees (palm oil and palm kernel oil). The
word palmitic is from the French "palmitique",
the pith of the palm tree. Butter, cheese, milk and meat also contain
this fatty acid.
top
|
----------------------------------------------------------------
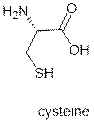
What is palmitoylation?
|
Palmitoylation is the covalent attachment of fatty acids, such as palmitic
acid, to cysteine residues of membrane proteins.
The precise function of palmitoylation
depends on the particular protein being considered. Palmitoylation
enhances the hydro-phobicity of proteins and
contributes to their membrane association.
|
|
|
Palmitoylation also appears to play a
significant role in subcellular trafficking of proteins between membrane
compartments, as well as in modulating protein-protein interactions.
top
|
----------------------------------------------------------------
What is peptide?
|
Peptides
are the family of short molecules formed
from the linking, in a defined order, of various α-amino acids. The
link between one amino acid residue and the next is an amide bond and is
sometimes referred to as a peptide bond.
top
|
----------------------------------------------------------------
What is peptide synthesis?
|
Peptide synthesis is the creation of peptides, which are organic compounds in which
multiple amino acids bind via peptide bonds which are also known as amide
bonds.
Peptides are synthesized by coupling
the carboxyl group or C-terminus of one amino acid to the amino group or
N-terminus of another.
Liquid-phase peptide synthesis is a
classical approach to peptide synthesis. It has been replaced in most
labs by solid-phase synthesis. However, it retains usefulness in
large-scale production of peptides for industrial purposes.
top
|
----------------------------------------------------------------
What is SPPS?
|
Solid-phase peptide synthesis (SPPS), pioneered by Merrifield, is
now the accepted method for creating peptides and proteins in the lab in
a synthetic manner. SPPS allows the synthesis of natural peptides which
are difficult to express in bacteria, the incorporation of unnatural
amino acids, peptide/protein backbone modification, and the synthesis of
D-proteins, which consist of D-amino acids.
Small solid beads, insoluble yet
porous, are treated with functional units ('linkers') on which peptide
chains can be built. The peptide will remain covalently attached to the
bead until cleaved from it by a reagent such as trifluoroacetic acid. The
peptide is thus 'immobilized' on the solid-phase and can be retained
during a filtration process, whereas liquid-phase reagents and
by-products of synthesis are flushed away.
The general principle of SPPS is one
of repeated cycles of coupling-deprotection. The free N-terminal amine of
a solid-phase attached peptide is coupled to a single N-protected amino
acid unit. This unit is then deprotected, revealing a new N-terminal
amine to which a further amino acid may be attached.
There are two majorly used forms of
SPPS -- Fmoc
and Boc.
Solid-phase peptide synthesis proceeds in a C-terminal to N-terminal
fashion. The N-termini of amino acid monomers is protected by these two
groups and added onto a deprotected amino acid chain.
Automated synthesizers are available
for both techniques, though many research groups continue to perform SPPS
manually.
SPPS is limited by yields, and
typically peptides and proteins in the range of 70~100 amino acids are
pushing the limits of synthetic accessibility. Synthetic difficulty also
is sequence dependent. Longer lengths can be accessed by using native
chemical ligation to couple two peptides together with quantitative
yields.
top
|
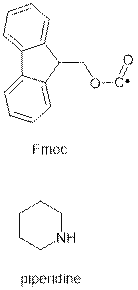
----------------------------------------------------------------
What is Fmoc
SPPS?
|
This method was introduced by Carpino
in 1972 and further applied by Atherton in 1978. Fmoc
stands for (F)luorenyl-(m)eth(o)xy-(c)arbonyl which
describes the Fmoc protecting group, first
described as a protecting group by Carpino in 1970. To remove an Fmoc from a growing peptide chain, basic conditions
(usually 20% piperidine in DMF) are used.
Removal of side-chain protecting
groups and peptide from the resin is achieved by incubating in
trifluoroacetic acid (TFA). Fmoc deprotection is
usually slow because the anionic nitrogen produced at the end is not a
particularly favorable product, although the whole process is
thermodynamically driven by the evolution of carbon dioxide. The main
advantage of Fmoc chemistry is that no
hydrofluoric acid is needed. It is therefore used for most routine
synthesis.
top
|
|
----------------------------------------------------------------
What is Cross-linker?
|
Cross-linkers are covalent bonds linking one polymer chain to another. In biology,
cross-linking has applications in forming polyacrylamide gels for gel
electrophoresis and in protein studies. Crosslinking inhibits close
packing of the polymer chains, preventing the formation of crystalline
regions. The restricted molecular mobility of a crosslinked structure
limits the extension of the polymer material under loading.
In most cases, cross-linking is
irreversible, and the resulting thermosetting material will degrade or burn
if heated, without melting. Once a substance is cross-linked, the product
is very hard or impossible to recycle. In some cases, though, if the
cross-link bonds are sufficiently different, chemically, from the bonds
forming the polymers, the process can be reversed. Permanent wave
solutions, for example, break and re-form naturally occurring cross-links
(disulfide bonds) between protein chains in hair.
A variety of crosslinkers are used to
analyze subunit structure of proteins, protein interactions and various
parameters of protein function. Subunit structure is deduced since
crosslinkers only bind surface amino residues in relatively close
proximity in the native state. Protein interactions are often too weak or
transient to be easily detected, but by crosslinking, the interactions
can be captured and analyzed.
An example is the the cleavable trifunctional Sulfo-SBED.
It has biotin covalently attached to a heterobifunctional reagent. In
addition to the biotin moiety, the second and third functional groups are
a sulfonated N-hydroxysuccinimide (Sulfo-NHS) active ester and a photoactivatable aryl azide, respectively. In addition, the linkage
containing the active ester has a cleavable disulfide bond.
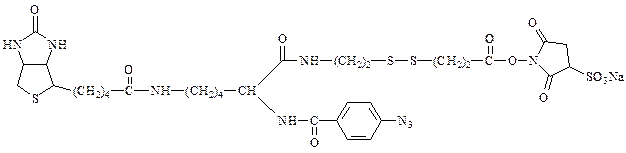
top
|
----------------------------------------------------------------
What is biotin?
|
Biotin, also known as vitamin H or B7,
has the chemical formula C10H16N2O3S
(Biotin; Coenzyme R, Biopeiderm), is a
water-soluble B-complex vitamin which is composed of an ureido (tetrahydroimidizalone) ring fused with a
tetrahydrothiophene ring.
|
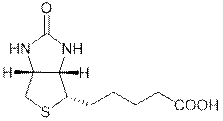
|
|
A valeric acid substituent is attached
to one of the carbon atoms of the tetrahydrothiophene ring. Biotin is
important in the catalysis of essential metabolic reactions to synthesize
fatty acids, in gluconeogenesis, and to metabolize leucine.
In the biology laboratory, biotin is
sometimes chemically linked, or tagged, to a molecule or protein for
biochemical assays. This process is called biotinylation.
Since avidins bind preferentially to biotin, biotin-tagged molecules can
be extracted from a sample by mixing them with beads with
covalently-attached avidin, and washing away anything unbound to the
beads.
For example, biotin can be attached to
a molecule of interest (e.g. a protein), and this modified molecule will
be mixed with a complex mixture of proteins. Avidin or streptavidin beads
are added to the mixture, and the biotinylated molecule will bind to the
beads. Any other proteins binding to the biotinylated molecule will also
stay with the beads. All other unbound proteins can be washed away, and
the scientist can use a variety of methods to determine which proteins
have bound to the biotinylated molecule.
Biotinylated antibodies are used to
capture avidin or streptavidin in both the ELISPOT and ELISA techniques.
top
|
----------------------------------------------------------------
What is Streptavidin?
|
Streptavidin is a 60,000 dalton tetrameric protein
purified from the bacterium Streptomyces avidinii.
It finds wide use in molecular biology through its extraordinarily strong
affinity for the vitamin biotin; the dissociation constant (Kd) of the biotin-streptavidin complex is
on the order of ~10-15 mol/L, ranking among one of the
strongest known non-covalent interactions.
top
|
----------------------------------------------------------------
What is Avidin?
|
Avidin is a glycoprotein found in the egg
white and tissues of birds, reptiles and amphibians. It contains four
identical subunits having a combined mass of 67,000-68,000 daltons. Each subunit consists of 128 amino acids and
binds one molecule of biotin. The extent of glycosylation is very high.
Carbohydrate accounts for about 10% of the total mass of avidin. Avidin
has a basic isoelectric point (pI) of 10-10.5
and is stable over a wide range of pH and temperature. Extensive chemical
modification has little effect on the activity of avidin, making it especially
useful for protein purification. Because of its carbohydrate content and
basic pI, avidin has relatively high
nonspecific binding.
top
|
----------------------------------------------------------------
What is Biotinylation?
|
Biotinylation is the process of covalently attaching
a biotin tag to a molecule or surface.
top
|
|
|
|